Riches of the Earth: the Beauty of Minerals. a free exhibition from 1 April to 7 October 2023.

This guide is intended as a source of interesting facts connected to the objects on display, but is not essential to viewing the exhibition.
But that which served as steps under our feet became in other places stalactites. The lava, very porous in certain places, took the form of little round blisters. Crystals of opaque quartz, adorned with limpid drops of natural glass suspended to the roof like lustres, seemed to take fire as we passed beneath them. One would have fancied that the genii of romance were illuminating their underground palaces to receive the sons of men.
“Magnificent, glorious!” I cried in a moment of involuntary enthusiasm, “What a spectacle, Uncle! Do you not admire these variegated shades of lava, which run through a whole series of colors, from reddish brown to pale yellow—by the most insensible degrees? And these crystals, they appear like luminous globes.”
“You are beginning to see the charms of travel, Master Harry,” cried my uncle. “Wait a bit, until we advance farther. What we have as yet discovered is nothing—onwards, my boy, onwards!”
Extract from Jules Verne
‘A Journey to the Centre of the Earth’1864
Content Links
(jump to section)
Introduction to the exhibition
The exhibition focuses on mineral specimens as natural wonders. They are displayed so that you can appreciate them as aesthetic objects in their own right.
In this guide to the exhibition we describe their physical qualities and origins, formed over thousands or millions of years, crystallising from liquids, often at great heat and pressure.

Colour and Form
All these specimens are from the Bath Royal Literary and Scientific Institution Collection and have been selected for their beautiful form and colour. They range from the glacial blue-green beryl, rainbow-hued opal, fiery red heulandite, lurid yellow sulphur, to the hexagonal prisms of aragonite, eccentrically-fused cubes of fluorite, and acicular (needle-like) crystals of Goethite.
One group demonstrates their curious ability to glow in strange colours under UV light, while another has been chosen for their ornate patterns exposed in cross section.
The Macro Photographs
Around the walls a series of macro photographs reveal the minute details of these minerals.
These details, normally beyond the reach of the naked eye, are indications of the chemical and physical conditions of the minerals’ formation.






History of the collection
Our collection of more than 2300 mineral specimens was donated by various 18th and 19th century collectors.
Mineralogy was one of many interests that academics, clergymen, landed gentry, and entrepreneurs with an interest in natural science, pursued with enthusiasm.
Their purpose varied: scientific study, industrial research, or the desire to create a private collection.
Early systematic classification was rudimentary because of a poor understanding of chemistry, so minerals were often grouped according to appearance.

Definition: Mineral
1. A naturally occurring inorganic element or compound having an orderly internal structure and characteristic chemical composition, crystal form, and physical properties.
— Dictionary of Mining, Minerals, and Related Terms
◆ Most minerals are compounds of two or more chemical elements. When a mineral is composed of just one, it is called a ‘native element’.
◆ Each year about 30–50 new minerals are described and one or two are discredited.
◆ As of January 2017 there were
5899
known minerals on earth.
◆ The physical properties of minerals include crystal structure, hardness, lustre and colour, and more complicated properties such as streak, fracture, cleavage and density.
◆ They have a consistent and characteristic internal atomic structure and a fixed chemical composition.
◆ Minerals can be smelted to extract metals or cut into gemstones. We ingest them in our food and water and make them into components for modern technology.
◆ When two or more minerals combine together, they form a rock. The number of combinations occurring produces a great many different rocks.
How minerals form
There is no one mechanism of mineral formation.
Different minerals form in different circumstances, though all minerals form either through cooling of molten material or precipitation from a saturated solution.

Piece of an amethyst geode
A geode starts life as a hollow bubble inside a layer of rock. As mineral-rich water moves on through, tiny crystals are left behind on the sides and over millions of years these grow inside the empty space
The ultimate source of all minerals is the cooling of molten rock or magma, which happens as it moves closer to the Earth’s crust.
The rate of cooling affects the degree of crystallisation and the size of crystals. An extruded magma, lava, cools very rapidly and is often amorphous, lacking significant crystallisation.
On the other hand a large body of magma, cooling slowly and at great pressure deep below the surface, forms large interlocking crystals, as seen in granite.
All rocks are composed of minerals
Igneous rocks, those formed directly from magma, erode to form sediments that are then deposited and compacted to form sedimentary rocks.
Deep burial and heating of sedimentary rocks may cause them to alter, and in some cases recrystallise. These are known as metamorphic rocks.
Hot fluids
Fluid passing through or across a rock may dissolve minerals and transport them as ions. When these solutions become saturated they re-precipitate as minerals.
The heat generated by magma often drives hot fluids through the surrounding rock and this can precipitate as mineral veins or druses (coatings or in-fillings of small crystals). Cool water, particularly if acidic, can also dissolve rock and precipitate minerals, this is how cave calcites form.
Crystals
Minerals form crystals when they have had the chance to grow unimpeded, with well-defined shapes dictated by forces acting at the molecular level.
During crystallisation, molecules cluster together to form nucleation points within a fluid and then grow concentrically. How large a crystal grows is determined by the rate of cooling or precipitation, and the space available for it to grow.
The largest known crystals are the selenite crystals of Cueva de los Cristales in Mexico, which reach
11 metres in length.
Arrangements of crystals
Thin section microscope slides of rocks viewed under cross polarised light reveal the different mineral crystals of which they are composed.
This is a thin section of gabro, a rock formed from slowly cooled magma.
Large crystals interlock with one another. The stripy grey, black, and white crystals are plagioclase feldspar, while the colourful crystals are olivine and clinopyroxene.
Gold and silver
The beauty of gold and silver, combined with their useful physical properties, have ensured their enduring appeal.
The low reactivity of gold means it does not tarnish and this, combined with its scarcity and ease of working, have made it greatly valued.
Silver is more durable, but tarnishes more easily and is not as rare.
Sylvanite, on display in the same case, is a mineral that contains both silver and gold in a compound with the lesser known element, tellurium.
It was first discovered in Transylvania and forms soft metallic crystals that tarnish when exposed to bright sunlight.
Gold
Most gold comes as a native metal. It occurs as nuggets or tiny flakes in the sands of river beds, in volcanic hot springs, in veins created by certain granites as they rise into the earth’s crust, and in metamorphosed river gravels.
Gold can be beaten to a thickness of 0.1 micron. A stack of one thousand sheets of 0.1 micron gold leaf is the same thickness as a typical piece of printer paper.

‘As rich as Croesus’
Because of his legendary wealth, the earliest gold coins have often been attributed to King Croesus, King of the Lydians in Western Anatolia (between 560-547 BC). Prior to that coins had been made of electrum, an alloy of gold and silver which occurred naturally in river sands.

The largest gold nugget found, the ‘Welcome Stranger’, measured 61 by 31 cm and was discovered in 1869 by two prospectors in Victoria, Australia. They were paid the equivalent of a million pounds in today’s money. The gold was quickly melted down and shipped to the Bank of England.
Silver
Unlike gold and copper, in nature silver rarely occurs in its pure metallic state. Silver had to be extracted from ores and this probably happened when heating copper deposits.
Even today, most silver is produced as a fractional by-product of the smelting of other metals such as lead – where for each ton of lead, a few ounces of silver are generated.
The Greek word for silver ‘argos’ and the Latin word ‘argentum’ are related to the Sanskrit term ‘arjuna’, meaning white or shining.

Silver has been used as a coinage metal since the times of the Greeks.
The need to find new sources of gold and silver ores helped fuel the ambitious conquests of the Macedonian kings, Philip II, and his son, Alexander the Great. This coin, a silver tetradrachm (320BC), was one of Alexander’s new silver coins which became the staple coinage of the Greek world.
Here, Zeus holds an eagle and sceptre.
A spectrum of colour
Minerals have long been collected for the quality of their rich and distinctive colours.
The internal arrangement of atoms within minerals determines all their chemical and physical properties, including colour.
Light interacts with different atoms to create different colours.
The orange and yellow tints of Jupiter’s volcanic moon, Io, are thought to be caused by the sulphur compounds deposited by active volcanoes.
The surface material on Mars contains abundant iron oxide – the same compound that gives blood and rust their reddish hue.
Ochre
Ochre – iron oxide – was the first ‘colour paint’, widely used from prehistoric times. The word refers to almost any natural earthy pigment, although it more accurately describes earth that contains a measure of haematite (iron ore). Unlike vegetable dyes, this pigment does not fade, which made the sources of red and yellow ochre very valuable in the past.

Prehistoric cave painting of a bison, Altamira, Spain. Museo de Altamira
Amethyst
This is the most precious form of quartz but its deep colour can fade in the light and also be artificially darkened by irradiation. Amethyst often forms the internal lining of geodes.

Sulphur
Sulphur is soft and very brittle. In the Bible, it is called brimstone. When burned, sulphur melts to a blood-red liquid and emits a blue flame and an acrid smell, evoking images of hell and giving rise to the term ‘fire-and-brimstone’ sermons.

Collecting sulphur from an Indonesian mine, photograph by Kondephy on Wikipedia
Pyromorphite
The name is derived from the Greek for fire (pyr) and form (morfe) because pyromorphite has an unusual behaviour — recrystallising after it has been melted.
Galena
Galena is a lead sulphide mineral. It was known as ‘potter’s ore’ and was an ingredient of the green glaze used in medieval pottery. With ageing, it released toxic lead compounds. Prolonged use of a contaminated vessel could cause blackened gums and, ultimately, death.

Medieval face-jug with green glaze
© The Trustees of the British Museum
Azurite
This mineral is produced as copper deposits weather. For medieval painters it was a major source of blue, although its intensity lessens over time. It was replaced by a synthetic colour ‘Prussian Blue’ in the 18th century.

In Michelangelo’s painting of ‘The Entombment’, the original blue of Mary’s robe, painted using azurite, (left foreground), has faded from blue to olive green.
Malachite
The intense green colour and beautiful banded masses of malachite give it a unique ornamental quality unlike that of any other stone. It is found in copper mines alongside its cousin, azurite.

The Malachite Cabinet, Kardriorg Palace and Art Museum, Tallinn, Estonia. Photograph by A.Davey on Flickr
Structural colour
Some minerals display
stunning plays of colour
when they refract light.
This iridescent optical effect
is the result of structural
colour– microstructures
within the crystals that
interfere with light – rather
than pigments that only
reflect certain wavelengths
of light.
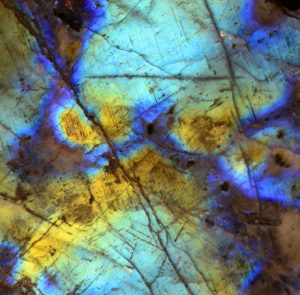
Labradorite
Labradorite is a mineral well known for its iridescent optical effect, technically referred to as ‘schiller’, meaning a reflection of light caused by thin planes within the mineral structure.
Precious Opal
Precious Opal has a regular structure composed of close fitting layers of spheres packed full of silica molecules.
This structure causes the diffraction and interference of light. The degree, order, orientation and spacing of the packing causes variations in the colour and intensity of the effect, giving each opal a unique play of
colour.
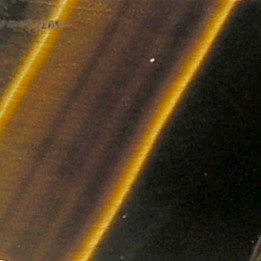
Tiger’s Eye
Tiger’s eye consists of parallel inter-growths of quartz and an altered form of asbestos.
The optical effect is called ‘chatoyancy’ (from the French ‘oeil de chat’, meaning ‘cat’s eye’) in which light is reflected at a right angle to the orientation of the fibres within the mineral.


Iron
The most common iron mineral is haematite, from a Greek word meaning ‘blood-red’.
Haematite, one of several iron oxides, is a more reddish variety of iron oxide and is the main component of red ochre. It is also the most important ore of iron, and was once mined in many places.
Unlike gold, silver, and copper, iron oxidises easily and so is not found as a native element. Pure iron is confined to meteorites, which formed from the iron and nickel cores of broken up planets.
Specular Iron
Haematite with a metallic lustre is called specularite or ‘micaceous haematite’. It can appear to be composed of shiny mica-like flakes, giving rise to the name micaceous iron, but the flakes are in fact haematite.
Kidney ore
When haematite precipitates in cavities, it has the opportunity to form an unrestricted habit known as ‘kidney ore’, for its similarity to the internal organ.
This type of chemically-precipitated hematite is often uncontaminated with sedimentary clay or rock inclusions and has a higher purity.
Ochre
Haematite was an important pigment for painters, being opaque and permanent. It could be mixed with a white pigment to produce a variety of pink colours that were used to paint flesh.
The iron molecules in red ochre act like compass needles. When daubed onto wet clay by the painter, they realign to magnetic north before they dry.
This has proved a great benefit in the dating of frescoes and cave paintings, as the earth’s magnetic field shifts over time.
Extra-terrestrial iron
Pallasites are a class of stony-iron meteorite formed of native iron and nickel in which olivine crystals are
suspended. They formed more than 4.2 billion years ago.
The mixture of material from which they are made comes from the core-mantle boundary of a small planet,
broken up by a massive impact.
They are part of the evidence that our own planet has a nickel-iron core
and a plastic mantle made up of olivine and pyroxene minerals.

Our very own pallasite
Only sixty-one pallasites are known on earth, and the BRLSI is fortunate to have a piece of the first known, the Krasnoyarsk pallasite, discovered by the German natural historian, Peter Simon Pallas (1741–1811).
On discovery in central Siberia in 1771, weighing 680kg, it was so obviously distinct from the local geology that it was transported to St Petersberg to be studied by Pallas.
Though not initially identified as such, this was the first object discovered on earth to be confirmed extra-terrestrial. The BRLSI specimen was donated to us by the Wiltshire antiquarian Sir Richard
Colt Hoare, as part of a collection of minerals from Siberia.
‘The First Meteorite’ Trail
Use our free walking trail App to imagine this object from outer space that is small enough to fit in your hand and is 4.2 billion years old. This trail explores ‘deep time’ and the very origins of our solar system through a story that connects 18th-century astronomers with druid temples and a Siberian forest. Once you have completed your journey to BRLSI you will be able to see this unique fragment from the earliest years of the universe.
Copper
Native copper, which is the metal occurring naturally, was one of the first worked by humans, being easy to fashion into tools and ornaments.
Native copper is uncommon. Copper
is usually found as compounds, either as the primary ore, chalcopyrite, formed at depth by crystallisation from hot liquids, or as a secondary copper mineral formed through weathering of chalcopyrite, particularly as a result of oxidation.

Today most mined copper is derived from sulphide ores not exposed to weathering.
However, the first copper minerals exploited were secondary copper oxides which form near the surface. The characteristic colours of these secondary copper minerals are easily
visible in the rocks in which they occur.
Malachite and Azurite
Malachite, a vivid green, and azurite, brilliant blue crystals, are often found
together.
Cuprite
Richer in copper and a more important ore, is cuprite, often called ruby copper because of its fine red colour.
Chalcopyrite
Chalcopyrite, a brassy looking mineral, is an important ore of copper, and one of several minerals dubbed ‘fool’s gold’ because it so readily deceived inexperienced prospectors in the past.
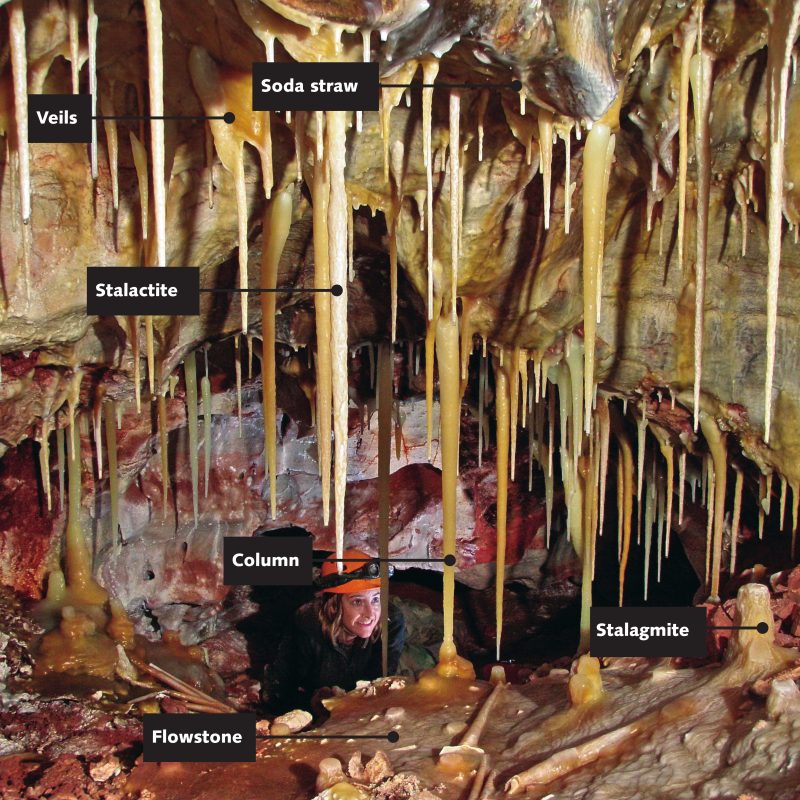
Cave formations
The spectacular, irregular formations found in caves are called speleothems. They are usually composed of the mineral calcite, having formed in areas where the bedrock is limestone.
Rain water passing through the soil becomes saturated with carbon dioxide and becomes weak carbonic acid.
This acidic groundwater dissolves the permeable limestone and becomes saturated with calcium and carbonate ions.
When the water makes its way into a cave ceiling, it hangs as a drop, then falls onto the floor, leaving behind a tiny deposit of calcite crystal.
Stalactites and stalagmites, cave veils, cave pearls, and soda straws are all types of speleothem. As they grow by only a fraction of a centimetre in a year, large ones take many thousands of years to form. Where the water supply is seasonal it may be possible to see growth rings similar to those of a tree trunk.
Soda Straws are thin-walled hollow formations. As water drips slowly from the roof of the cave, it deposits a microscopic ring of calcite crystal. These rings continue to build and can form straws many centimetres long.
Stalactites grow downwards from the cave roof. Nearly all stalactites start their life as a straw, until the straw becomes blocked with calcite or impurities. As the stalactite starts to develop, it thickens over time from the solution which runs down its outer surface.
Stalagmites are solid dripstones that grow upwards from the cave floor as drops of water fall from the stalactites overhead.
Columns develop from stalactites or stalagmites which have extended from floor to roof.
Veils form when water trickles down a rockface, depositing a narrow strip of calcite that eventually becomes a thin sheet, growing at an angle from the wall. The curious folds occur when the initial trickle moves from side to side in its downward path along the rockface. Coloured banding is caused by minerals such as iron oxide in the solution.
Flowstones are caused by flowing water covering the original rock or mud floor, leaving a film of calcite. Sometimes the lower portions hang free, making a fringe or shawl of stalactites.
Cave Pearls are spherical deposits of calcite which have developed around a nucleus such as a tiny pebble or grain of sand. Flowing water moves the grains about and they gather concentric layers of calcite.
Silica
Quartz is the crystalline form of silica (silicon dioxide) and is the most common mineral in the earth’s crust.
The most varied of all minerals, it occurs in many different forms, habits, and colours.
The sand on the beach, the circuits in mobile phones, and the glass in windows are all made of silica.
Frozen crystal?
The root for the word ‘crystal’ comes from the Greek word ‘krystallos’, meaning ice.
Among the ancient Greeks there was a belief that clear quartz was ice frozen so deeply that it could never melt.
Rock Crystal
Pure colourless quartz is called ‘rock crystal’, although it can be artificially irradiated to alter the colour and passed off as smoky quartz.
Quartz can occur in a range of colours from pink to brown to the deep purple of amethyst, depending on the number and type of impurities in its structure. The colour appears long after the crystals have grown and it can take millions of years for a deep colour to form.
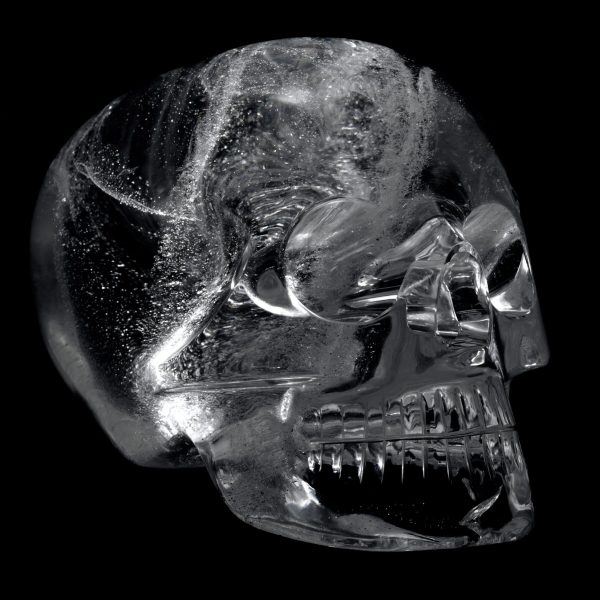
Rock crystal skulls, supposed to be Mesoamerican, like this one from the British Museum are now thought by many to be fake pre-Columbian artefacts made in Europe in the 19th century.
© The Trustees of the British Museum


Chalcedony
By strict definition both chalcedony and opal are mineraloids, as they do not display crystallinity.
Chalcedony is a microcrystalline silica; its structure gives it a waxy lustre and a very different appearance to the chemically identical quartz.

Opal
Opal is also a microcrystalline silica, but in this case it is hydrated, having up to 21% water by weight.
The presence of water in the structure of precious opal creates beautiful optical effects. Opal gemstones become more brilliant in colour when warmed; wearing an opal keeps it hydrated. Opal that becomes too dry is brittle and loses its unique play of colours.
Polished sections
From the late 18th century the demand for commercial ores to refine into lead, silver, copper, and iron grew rapidly. Mining activity often exposed unusual mineral specimens. They were eagerly sought for personal collections.
While minerals were increasingly being studied as the products of the laws of chemistry and physics, the connoisseur treasured them as objects of beauty and inspiration.
Polished sections revealed the inner beauty and random patterns that nature created.
Jasper
Jasper, which can have a strikingly bright colour, is a mixture of silica minerals including quartz and chalcedony.
The form displayed here is jaspalite, or riband jasper, which is usually associated with banded ironstone rocks.
The red and green colours come from two different forms of iron oxide in marine sediments. They were deposited up to 2.7 billion years ago, during the time when oxygen was first produced by cyanobacteria. The spike in oxygen caused dissolved iron in the oceans to be precipitated out and deposited alongside the shells of siliceous microorganisms.
Over time this was altered by heat and pressure to form a mineral.

Roman Jasper intaglio featuring the god Harpocrates riding a lion, from the Metropolitan Museum of Art
Agate
Agate is the most varied and popular type of chalcedony and forms in cavities.
The layers are laid down in stages, creating bands of alternating colour, each with a unique pattern based on the cavity shape.
If the cavity is only partially filled, the hollow void often has crystalline quartz growths on its innermost layer.

Chinese Agate cup and saucer,
Qing Dynasty (1723–1735), National Museum of the Château de Fontainebleau, France
Blue John
Blue John is a semi-precious mineral, a fragile form of fluorite with bands of a purple-blue or yellowish colour. Its only source is in Britain, at Castleton in the Peak District.
Its decorative qualities made it highly valued by wealthy 18th century collectors. Georgiana, Duchess of Devonshire, who had her own mineral collection, acquired some fine Blue John vases for Chatsworth House.
When is a mineral not a mineral?
Organic hydrocarbons, like coal and jet, are not strictly minerals and yet they are often considered ‘mineral resources’.
Mineraloids
Coal and jet are composed predominantly of carbon, but unlike crystalline forms of carbon, graphite and diamond, they are not pure enough to be considered true minerals, but ‘mineraloids’. A mineraloid lacks crystallinity and a well-defined chemistry.
Jet
Jet is fossilised wood, traditionally used in jewellery as a gemstone. Whitby Jet, as seen in the display specimen, is Early Jurassic in age and comes from trees closely related to the living fossil, the monkey puzzle tree (Araucaria).
It became fashionable in Victorian times when Queen Victoria wore Whitby jet as part of her mourning dress, after the death of Prince Albert.
Coal
Coal is also fossilised plant material, commonly used as a fuel. Coal may not be aesthetically pleasing, but when a thin layer of plant material is preserved as a coalified layer, the resulting fossils can be very beautiful.
The plant fossils on display both come from the Radstock Coal Measures and are 305 million years old.
Asterophyllites equisetiformis was a giant relative of modern horsetails, growing as a tree up to 30 metres tall. Pecopteris plumosa was a tree fern whose fronds are frequently preserved separately from the rest of the plant.
Fluorescent minerals
When illuminated with ultraviolet light, certain minerals glow with an amazing array of vibrant colours. The UV light causes these minerals to temporarily emit visible light of various colours, a property known as ‘fluorescence’.
When UV light shines on a crystal, it imparts energy to electrons within the crystal’s atoms. Each excited electron jumps momentarily away from the nucleus of its atom, then falls back.
When the electrons drop back, they release bursts of visible light.
This light released is different from the wavelength of ordinary daylight and causes a temporary colour change of the mineral in the eye of a human observer. This ‘glow’ continues as long as the mineral is illuminated with light of the proper wavelength.
You can see from the display that some minerals fluoresce more strongly, some fluoresce only a single colour, and others have multiple colours.

The prismatic crystals of apatite appear colourless under normal light
Under UV light they glow green
Crystallisation
Most minerals develop as crystals, for this is the normal condition of a chemical substance when it solidifies from a liquid or a gas.
As the material solidifies, molecules within the liquid pull together in a pattern, the atoms arranging themselves in a regular network.
Crystal Size
The way a crystal looks – the number and orientation of its faces – is a function of its molecular structure and the environment in which the crystal solution solidifies.
Temperature, pressure, chemical conditions, and the space available, all affect growth.
Crystal Twins
When two or more crystals intergrow, they are known as twins. Adjacent parts interlock so that some planes of the crystal structure are shared.
The birth of new minerals
Over 200 new mineral species, (around 4% of all known minerals) have come into existence through human activity, mostly in the last two centuries.
Certain of these activities may unwittingly create an environment where chemical reactions can take place between materials that might otherwise not come into contact.
Mines are a particularly productive environment, but new minerals have been found inside smelters, old geothermal piping and on the surfaces of archaeological artefacts.
There is even one called calclacite, which occurs when historic mineral specimens, stored in the oak drawers of museum cabinets, react with tannins in the wood.
Compared to the billions of years it took for combinations of elements to form the minerals we know, these new minerals are now being made faster than at any time in earth’s history.
For the next generation of mineral collectors, this potential offers intriguing opportunities.
The names of some human-created minerals:
- tinnunculite
- andersonite
- simonkolleite
- metamunirite
- chalconatronite
- abhurite
- bobcookite
- calclacite
- elyite
- widgiemoolthalite
- acetamide
- hoelite
- kladnoite
Credits
Written and designed by Jude Harris
Edited and uploaded by Matt Williams
Proof read by Rosemarie Davies
All images on this page, other than those from the BRLSI Collection, are either in the public domain or are attributed under the conditions of a creative commons licence. For practical purposes, the images herein should not be reused in order to avoid infringement of any copyright that may exist.